
Energy level diagrams can be drawn for molecules as well as for atoms. In other words, energy level diagrams depict the energies of molecular orbitals (MOs) in a molecule. Representation of energy level diagrams:Įnergy level diagrams are the pictorial representations of energy levels of electrons in an atom or molecule. The possible values an electron can have for any given energy level are also quantized. The energies of photons emitted by an atom are always quantized, which means they can only have certain values. The energy of a photon is equal to the difference in energy between the two energy levels involved in the transition. The most important feature of an energy level diagram is the line between two energies called an “energy transition.”Įnergy transitions correspond to photons being emitted or absorbed by an atom.
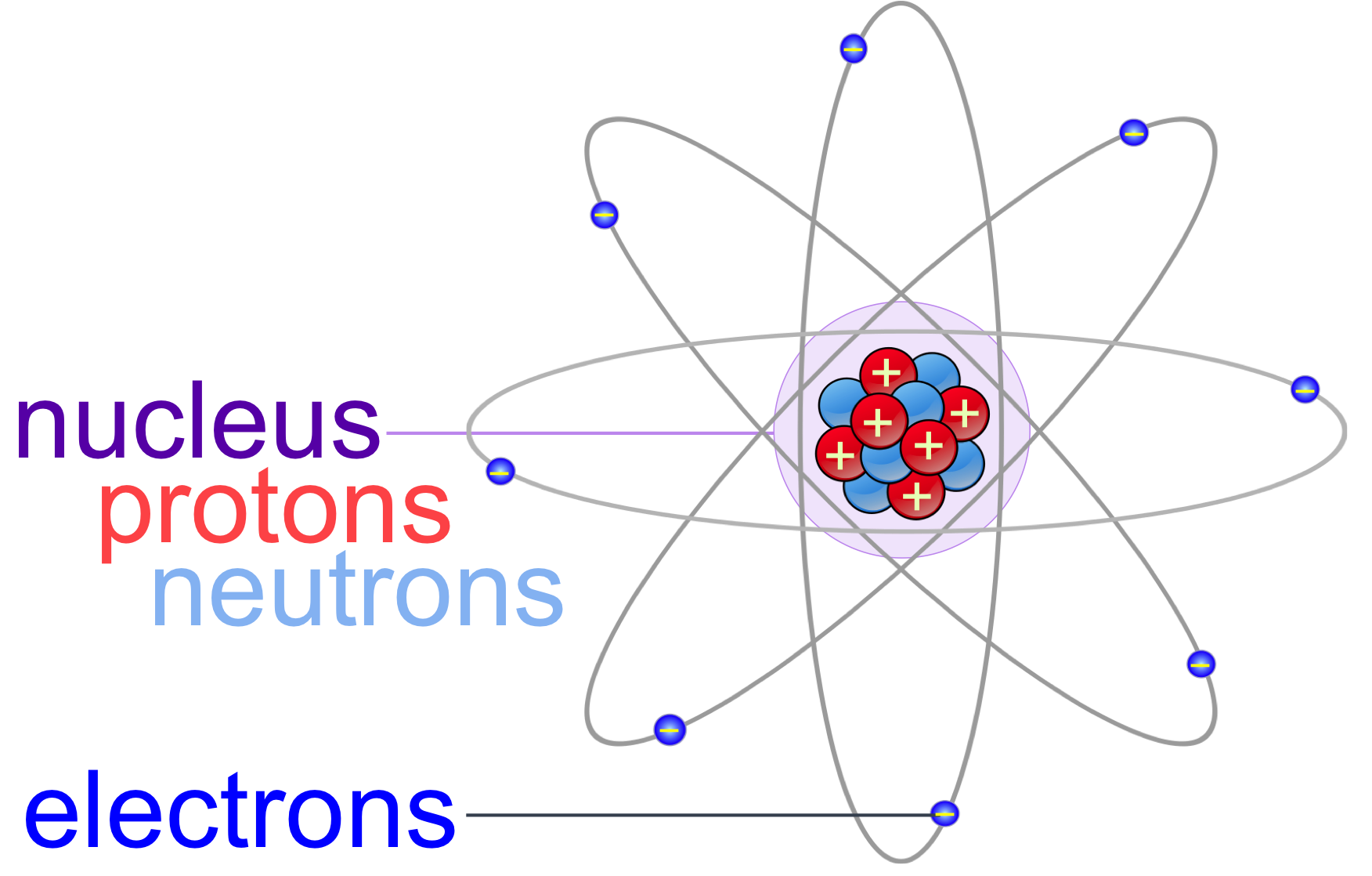
In an energy level diagram, the horizontal axis represents the energy of an electron and the vertical axis represents the number of electrons present at that energy. To understand the light emitted by atoms, we need to understand energy level diagrams. The horizontal axis represents the orbital energy, and the vertical axis represents the total energy of the system. The blue line represents the ground state energy, and the red line represents the excited state energy. The molecular orbitals are shown as horizontal lines, and the energies are shown as vertical lines. This type of diagram shows the energies of the orbitals in a molecule. The most common type of energy level diagram is the molecular orbital energy level diagram. Stay tuned for more! What is an energy level diagram?Īn energy level diagram, or energy band diagram, is a graphical representation of the allowed energy states of a system. In this blog post, we will take a closer look at the energy level diagram of the hydrogen atom and explore how it helps us understand atomic structure and bonding. The hydrogen atom is a good example to start with in order to gain a fundamental understanding of energy level diagrams. That third orbital is our outer shell or valence shell, meaning that sodium has one valence electron.A clear depiction of the energy level diagram is important to understand the various states and transitions of energy in an atom or molecule. That means two electrons will go in the first shell, eight in the second and one in the third. Using the example of sodium started above, we know that sodium as a neutral atom will have 11 electrons. Two electrons will go in the first shell closest to the nucleus and eight can go in each subsequent shell. These will represent the shells in which the electrons orbit the nucleus. In the last step you will need to draw circles around the nucleus. Here you will write the number of protons and neutrons as shown below in this example using sodium (Na) To set up the diagram, you will need a circle in the middle. This is because protons are positively charged and electrons are negatively charged so cations will have more protons than electrons and vice versa for anions.
#SIMPLE DIAGRAMS OF ATOMS PLUS#
For anions (negative ions), the number of electrons will equal the number of protons plus the absolute value of the charge. For cations (positive ions), the number of electrons will equal the number of protons minus the charge. A neutral atom will have the same number of electrons as protons.

To find the number of electrons you have to compare the charge to the number of protons. This is because protons and neutrons both weigh 1 atomic mass unit (amu) and electrons weigh essentially 0 amu. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. The number of protons is the atomic number.


 0 kommentar(er)
0 kommentar(er)
